Radical transformation in the global biopharma industry may bring about an awaited leap forward of the Israeli biopharma industry. How do we realize Israel’s competitive advantage in an age of personalized medicine?
Introduction: The future of medicine starts in Israel
Dr. Kira Radinsky
Israeli tradition has been known for its deep belief in Tikkun Olam – improving the world and building a model society. Israel has long shown its dedication to social justice through its healthcare systems, providing care for any and all while digitalizing data at an accelerating rate. This data has been accumulating for 20 years and serves today as a groundbreaking change in the way healthcare can be provided to Israelis.
The AI preventive care revolution:
For the first time in history, a medical system can study its patients statistically for long periods of time and provide insights originating from big data to better predict diseases. The prevention of illness is playing an ever-larger part in the Israeli medical ecosystem, as startups and homegrown initiatives of the health organizations are building digitalized solutions to intervene and protect people from chronic disease as early as their birth. Clalitnational health service providers has been developing tools to monitor diseases and predict acute myeloid leukaemia risk, while MaccabiIbidpresented an AI system that is able to predict the presence of colon cancer from a simple blood test.
The AI personalized treatment:
The wealth of data is opening the possibilities for true personalized medicine. Maccabi has been building tools to personalize drug treatment for hypertensive patients. Based on the large data accumulated they are able to present real-time recommendations to the medical provider suggesting which drugs will best affect the specific patients at hand.
Numerous solutions and companies have also focused on genetic-based personalized treatment. Armed with comprehensive genomic databases, Israeli companies like FDnA, leverage AI to detect physiological patterns that reveal disease-causing genetic variations. These can change the game for personalized treatment and preventive medicine just by looking at the patient’s face.
Medical Breakthroughs based on Big Data:
The data accumulated by this eco-system provides medical insights that can serve not only Israeli citizens, but also provide real breakthrough in medical discoveries. Several studies have now been initiated to create algorithms to identify drugs that have association and causation with negative and adverse symptoms.
Decision Support Systems:
With the growing shortage of doctors and medical staff in the world, the wealth of anonymized patient data accumulated in the Israel ecosystem serves as a basis for developing automated diagnosis tools that represent the future of medicine. One such example is the automated radiologist solution Developed by Israeli companies such as ZebraMed and AIdoc, which provide a support tool for medical providers via data and diagnostics.
Revolutions come from need, and the medical system is in need. The growing, aging population with diminished access to medicine limits our chances for a fair society, where each person has the same right to live. Israel has all the resources to lead this change in the world and to bring Tikkun Olam.
Dr. Kira Radinsky, Director of Data Science, eBay and Israel’s Chief Scientist
The medical automation and information revolution described in the introduction is giving rise to dramatic changes in all branches of medicine such as the global biopharma industry, which is hungry for innovative technologies. Personalized medicine, in particular, is becoming increasingly pervasive. Innovation in genetics and biology is being integrated with developments in big data and AI (Artificial Intelligence), revolutionizing the entire drug development process. This trend is blurring the boundaries between ‘classical’ biopharma and IT (Information Technology).
Israel’s many years of excellence in biomedical science have yet to translate into the creation of a mature local biopharma industry. The trend outlined above creates an opportunity for the Israeli biomedical science with its lead in IT to develop a global technology center for personalized medicine. In this chapter, we will describe personalized medicine’s effect on the global biopharma industry, and we will present the Innovation Authority’s vision to harness it as an engine of growth for Israel’s biopharma ecosystem.
The global biopharma industry is undergoing a profound transformation
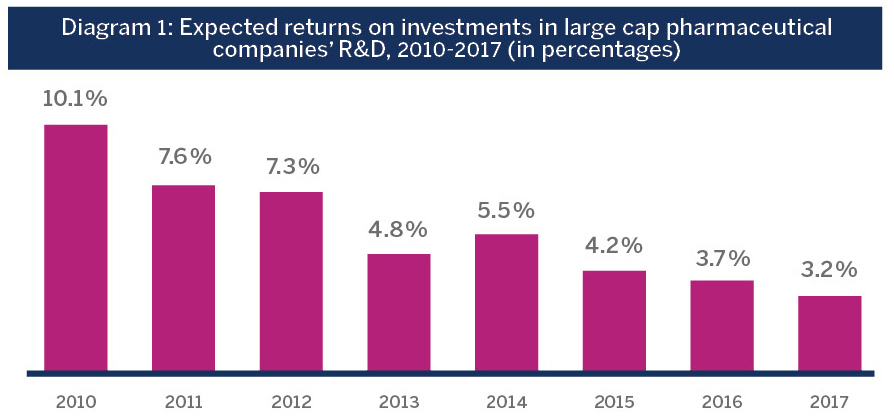
In recent years, the traditional business model for drug development, which has dramatically improved the health of humankind over the past century and laid the groundwork for global biopharma giants, has reached a crossroads. There has been a decline in the number of late- stage R&D pipeline developments in large cap biopharma companies, with a parallel increase in development costs. This is evidenced in steadily declining returns over the past decade on R&D investments in the global biopharma industry from 10% in 2010 to roughly 3% in 2017.Deloitte. (2018). A new future for R&D? Measuring the return from pharmaceutical innovation 2017
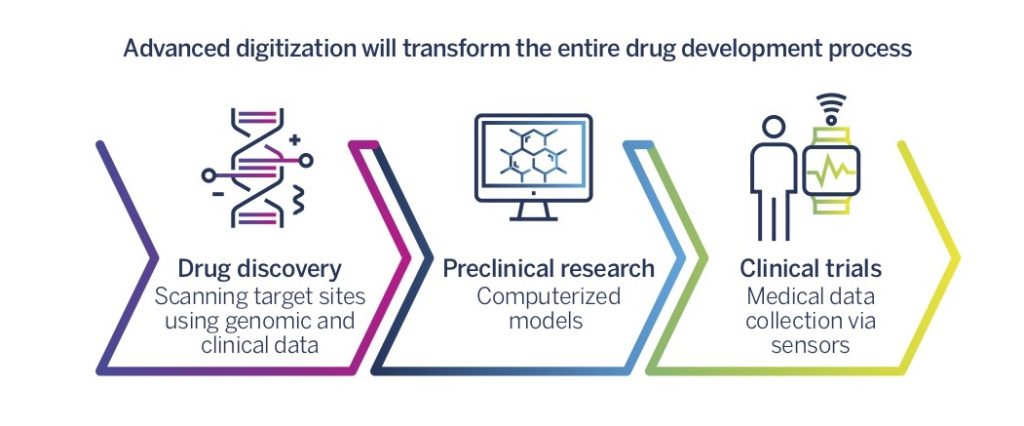
First, the drug discovery phase is expected to change significantly. Medical big data analysis derived from a range of sources, and especially the linking of medical records (EMR/EHR), genomic information, and functional protein-centric data (OMICS data), will allow researchers and companies to identify target sites (for example, a problematic protein in the cell) in order to discover new drugs with more ease and speed. At the same time, revolutionary CRISPR technology which enables genome editing and repair, has the potential of becoming a new, powerful tool for identifying target sites for new drugs by providing an innovative method to identify genes and proteins that cause or prevent disease .Scott, A. (2018, March 7). How CRISPR is transforming drug discovery
Second, new drugs will be developed and manufactured with more precision, which would ensure optimal safety and efficacy. Computerized models of biological processes, in particular, will be able to create information that could help in passing through the preclinical phase of drug development, in designing and conducting better clinical studies, and possibly in replacing some of the clinical data required for drug approval. Innovative drug manufacturing technologies, such as 3D-printing of drug components, could also help control new drugs’ toxicity in a manner that would facilitate their entire development and market-entry processes.
Third, advanced information technology will transform the clinical trial phases. Clinical development currently takes an average of 7.5 years and costs hundreds of millions to billions of dollars. Advanced information technology will significantly streamline this process. It will allow the selection of the most suitable trial participants using genomic and clinical data derived from a variety of sources (big data), allowing the monitoring of trial participants, using several types of sensors that transfer electronic data on a range of physiological measures. Biopharma companies will be able to examine the effect of drugs after they are on the market and thus reduce the expense of the clinical trial phase.The FDA-directed paradigm shift for drug approval has made information gathering on the action of new drugs in the ‘real world’ – not in the context of controlled clinical trials – critical
The technological changes outlined would generate significant regulatory changes. The ability to develop a drug suitable for a particular population, to ensure its safety, and to prove its efficacy with precision, is paving the way for faster, smarter, and cost-effective approval processes. Regulatory bodies across the globe are bracing for this trend, and are becoming open to new models for testing drugs that correspond with the new era of personalized medicine. For example, the FDA, the most authoritative regulatory body in the field of biopharma, has launched a program for the fast-track approval of cancer therapies, and will enable the harnessing of new technologies for data collection in clinical trials, such as physiological data collected from wearables.The Economist. (2018, March 24). FDA Wants to help unproductive drug makers
Israel can be a leader in the era of personalized medicine
These technological changes are expected to be a turning point for the global biopharma industry; they are also creating an opportunity for a long-anticipated Israeli breakthrough, thus spurring a new multi-disciplinary ecosystem in the local biopharma industry.
Over the years, Israel’s biopharma industry has not been meeting its extraordinary scientific potential: Most drugs discovered in Israel have been developed overseas by foreign companies,Teva’s Copaxone is the exception and the Israeli economy has lost lucrative business and high-quality jobs. Kite Pharma’s huge exit in 2017, valued at roughly $12 billion, is a clear example of this phenomenon. Its products are based on scientific developments at the Weizmann Institute, and the company was founded by a former Israeli; however, its operations are located solely in the US. While a number of innovative Israeli biopharma companies that launched operations in the past couple of decades have reached advanced development phases, most have yet to achieve considerable sales.
Nonetheless, recent changes in the local industry are pointing to a positive momentum. Israel’s biopharma industry is currently comprised of roughly 200 companies,IVC data with about 15 new ones being added to the list every year.IVC data – the average in 2012-2017 Investments in the field have seen a significant increase in recent years, and the average funding round has grown threefold (see diagram 2). In 2012-2017, investments by Israeli venture capital funds in the industry grew by 400 percent. Among other reasons, this increase reflects the new funds that have joined the field, and the establishment of additional investment bodies, such as FutuRx funded by the Innovation Authority.
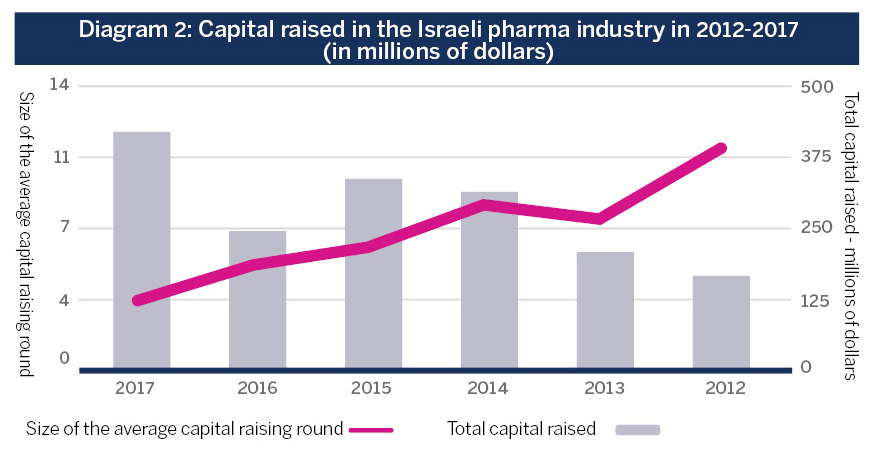
Source: IVC
The era of personalized medicine could potentially present a breakthrough for Israel’s biopharma industry, based on a number of assets that give it a unique competitive edge. One such key asset is Israel’s longstanding scientific excellence. Developments that emerged from Israeli academia are responsible for eight innovative drugs that were sold for a total of roughly $40 billion:As of 2014 Israel’s scientific community excels in cancer research, immunology, and the research of degenerative diseases – fields that are currently a focal point for the development of personalized medical therapies. Added to these are Israel’s unique genomic and medical data sources. Israel is comprised of a variety of singular population groups located within a small geographical territory. This makes it a ‘genomic goldmine,’ and EHR (Electronic Health Records), which have been in use in Israel since as early as the 80s, cover the overwhelming majority of the population. Israel’s lead in information technology is allowing the leveraging of medical and genomic data, and its advanced healthcare system is providing a platform for productive collaborations.
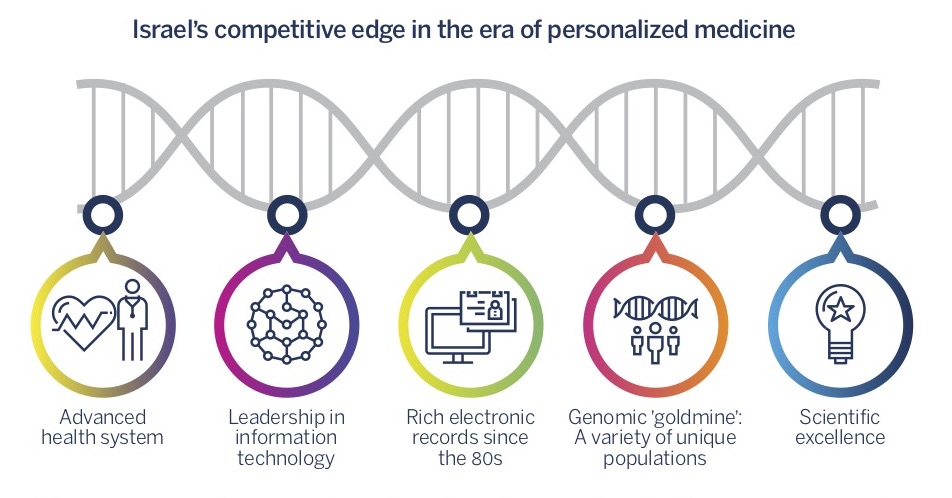
This competitive edge is significant throughout the entire drug development process, and is especially crucial in the drug discovery and clinical development phases. To capitalize on this advantage, it is important to reinforce the connection between relevant fields of research, to make the required information and research infrastructure accessible, and to encourage investment in groundbreaking technologies – biological, computational, and integrated. At the same time, the importance of ‘traditional’ technological and business skills that are still critical for the biopharma industry should not be underestimated. Consequently, while bolstering the outlined advantage, it is important to examine the factors that are currently impeding the success of Israeli biopharma companies, and to remove the pertinent obstacles.
Drug discovery
In personalized medicine, drug discovery makes use of scans of huge genomic and clinical data sets in order to identify new target sites that are specific for certain population groups. Thus, at this stage, the integration of Israel’s genomic and clinical data with computational capabilities carries great potential.
In the Israeli industry, there are already several companies using this drug discovery method. Ayala Pharmaceuticals developed a personalized treatment for a specific group of cancer patients carrying a genetic mutation, based on a diagnostic marker that is identified through clinical and genomic data. Another example is ImmPACT-Bio, which the Israel Innovation Authority supported through FutuRx incubator. It develops a CAR-T technology – a treatment in which a patient’s T cells are changed so they will attack cancer cells. The treatment will be personalized to each patient based on bioinformatics tools and databases of patients’ samples. A third example is CytoReason that aims to discover drugs using machine learning models applied to biological data of the immune system. These industry operations are joined by academic multidisciplinary research centers, such as the Israel national Center for Personalized Medicine at the Weizmann Institute that incorporates bioinformatics in drug discovery.
Recently, this activity in academia and industry received backing via a number of government measures. One such measure is the establishment of the Mosaic Project. The initiative, established in collaboration with the Innovation Authority, the Ministry of Health, PBC-CHE, and the Ministry for Social Equality, has led to the establishment of new genomic and clinical data infrastructure, to benefit both academic research and the development of products and services in the industry.
At the same time, the Innovation Authority, with joint funding by the national Digital Israel Initiative in the Ministry of Social Equality, is presently establishing a users’ association for digital health, which will serve as a network of medical data infrastructure to be shared with the industry. The stated objectives of the association, which will include startups, mid-to- large cap companies, as well as multinational companies, are to share new and existing medical data, to make the data accessible, and to establish regulatory infrastructure and information security. The program will work in collaboration with Israeli entities – both academic and clinical – as well as international entities. Both the Mosaic Project and the users’ program are slated to be a quantum leap in the field of medical data in Israel, to further advance existing relevant companies and to aid in the establishment of new companies.
In addition, since many breakthroughs in drug discovery are emerging from academia, it is important to ensure that processes for knowledge transfer from academia to industry occur correctly. Over the course of the past year, the InInnovation Authority closely consulted with industry and academia in an effort to map obstacles in the growth of the biopharma industry, and learned that the translational research phase, meaning the process of advancing from scientific discovery to launching of commercial drug development, is lacking. In particular, the process is lacking the early involvement of a business-industrial body specializing in the filtering of academic projects, in designing appropriate trials, and in laboratory-industrial development. The Innovation Authority, in collaboration with all relevant entities, is currently formulating a draft to revamp the process of translational research in Israel. Among other steps, the Authority will improve the testing and filtration mechanisms of technologies that emerge from basic research at their applicable research phases, and will attract international translational research experts to Israel, to encourage the flow of knowledge to local industry in the field.
Clinical trials
The aforementioned global trends in the field of clinical trials enable Israel to leverage its competitive edge for the success of its drug development companies, for the establishment of an entire ecosystem that supports clinical trials in Israel, and for making Israel a global focal point for clinical trials.
Currently, for Israeli biopharma companies, the transition from preclinical and early clinical development phases to the phase of proving drug efficacy in patients is commonly referred to as the valley of death. Due to funding difficulties that companies face, along with a shortage of experienced regulatory process managers, the clinical data created often does not satisfy the regulator. However, changing trends in the design and performance of clinical trials across the globe provide an opportunity for improved accomplishments for Israeli startup companies at this phase. A calculated selection of the trial population based on clinical and genomic data, along with regulatory openness for faster approval of personalized therapies, especially for cancer, will enable companies to pass each phase with far fewer patients, and will enable a larger portion of the clinical trials to be conducted in Israel. This means that Israeli biopharma companies that are able to harness these evolving trends could reach the market with fewer costs at a faster pace.
The aforementioned steps for making genomic and clinical data accessible to the industry are thus slated to help biopharma companies in clinical trial phases as well – especially when selecting the trial population. In addition, the Innovation Authority will increase its support in early clinical phases of effective, smart trials that are in line with the global trend.Consultation with the industry showed that government support of companies in early clinical phases is more critical and more effective than it is at more advanced phases The Innovation Authority will also create incentives to attract global experts in the management of regulatory processes to the Israeli ecosystem.
At the same time, global demand for advanced information technology that supports clinical trial phases, and the need to test personalized therapies on specific populations, could lead to the emergence of an entire ecosystem that supports clinical trials in Israel. Israeli biopharma companies would also benefit from this trend, because it would lead to the formation of a local body of knowledge on conducting clinical trials. There are already several companies in the Israeli industry that have recognized the need for advanced technological solutions to test the effects of drugs, and are throwing their hat in the ring. Pilltracker, for example, has developed an electronic device that enables the precise monitoring of clinical trial participants to ensure drug compliance, while updating participants on dose changes and receiving ongoing feedback from them. Data2Life is riding the global wave of analyzing the effect of drugs after market penetration (real-world data). The company aggregates data from patients through several channels: social media, medical monitoring devices, medical records, clinical trial data, and other sources. It uses this data to gain insight on the efficacy and side effects of drugs via machine learning and natural language processing.
Israel could also become a hub for conducting the actual clinical trials. High-quality research hospitals, advanced medical care, experience in conducting trials, and the small geographical area of the country make it relatively easy to recruit patients. As such, Israel is already attractive to global biopharma companies. For example, in 2017, companies submitted over 1,500 requests to conduct clinical trials in Israeli hospitals.Summary of 2017 and a comparison to 2014-2016, May 15 2018, The Ministry of Health, approvals by the Committee for Communications with Commercial Companies The penetration of personalized medicine and digitization trends in clinical trials could bolster Israel’s standing in the field. First, the variety of unique population groups in Israel makes it very attractive for conducting trials on personalized therapies based on genetic mutations (Parkinson’s patients in Israel). Second, being a leader in information technology that supports clinical trials will allow Israel’s ecosystem to provide innovative services to biopharma companies that will perform clinical trials in Israel.
In conclusion, the Innovation Authority sees personalized medicine as a potential turning point for the Israeli biopharma industry. Sweeping changes in the biopharma industry worldwide are creating an opportunity for breaking down barriers between the world of classical biopharma and the ICT industry, and for the emergence of new players and experts. We should harness the global technological trends and develop a multidisciplinary ecosystem that would be propelled by groundbreaking research, will make optimal use of existing genomic and clinical data in Israel, will leverage advanced information technology, and will ensure that the future of the global biopharma industry will begin in Israel.
