In the distant past, medicine relied on plant extracts of a simple kind. But these days, new botanical drugs are in demand. Coming up with them is an important scientific challenge. We introduce two Israeli biotech companies who are successfully meeting that challenge with cutting-edge development efforts.
In Israel’s Arava region, a company called Algatech is tapping microscopic algae for a powerful antioxidant called astaxanthin. More than half way across the country, in Yavne, a company called MediWound is extracting enzymes from pineapple stems to make unique products that can treat burns and chronic wounds. What do the two companies have in common?
Both are groundbreaking Israeli biotech companies that successfully develop and manufacture original drugs from plant life and market them worldwide – all with the assistance of the Israel Innovation Authority.
Dr. Zeev Even-Chen, a member of the Authority’s array of professional evaluators, explains: “Unlike conventional drugs, the medications we call ‘botanical drugs’ are usually a mix of substances. Some of the substances are plant extracts, but not all of them are. The elements typically act in synergy – that is, in combination – to make the active ingredients more effective.”
Today in the western world, stringent regulations require manufactured drugs to show no significant variation over time in their characteristics or their effect. Additionally, the manufacturing process must meet the pharmaceutical industry’s accepted standards as codified in the guidelines of the FDA (the American Food and Drug Administration). And just like synthetic drugs, plant-extracted drugs also need to be proven effective against a particular disease.
Supplement versus Drug: What’s the Difference?
Traditional medicine passed down verbally from generation to generation through centuries of history, uses plant-based substances to treat wounds, pain, and disease. But our ancestors didn’t understand the scientific complexities involved. They didn’t know what quality or ingredients the plant possessed that cured the patient of what ailed them, or how it happened. If a particular plant was found to relieve a headache or stomach ache, for example, people just used it.
The situation nowadays is entirely different. Developing a botanical drug based on traditional medicine, or based on a particular plant material that is known to contain a family of beneficial substances, requires attention to many details. A company has to know how to formulate the drug correctly, dispense it accurately, and keep it stable. Not to mention patenting it, respecting regulations, and being economically realistic.
Plant-sourced substances can be used to promote human health in three different ways:
- As part of our nourishment (a health food)
- As a food supplement
- As a drug
For each category, the supervision is distinct in both scope and regulations.
While health foods are relatively simple to understand, the difference between supplements and drugs can be more complicated. When plant products are marketed as dietary supplements, they come with no specific medical guidelines. They claim only to be “beneficial to health” in a general sense, and like any other food, they simply need to provide proof that they aren’t toxic. Dietary supplements do not, and must not, come with a promise to heal any illness or disease.
On the other hand, if a product is approved as a botanical drug, it comes with clear medical guidelines for use just as any other approved drug does.
Regulation lays down absolute restrictions for botanical drugs: Firstly, since such drugs are a mix of substances, each lot must be tested for compliance with specifications. And there must be a sort of ‘fingerprint’ showing which lot each purchased dosage belongs to.
Also, the FDA requires controlled growing conditions —kept stable artificially — because as seasons change and growing conditions vary, the same plant may not always yield an identical drug. If left to nature, the plant won’t always have the same content and concentration of active and inactive substances.
These two demands can place a burden on researchers and entrepreneurs who develop botanical drugs. According to Dr. Even-Chen, the number of FDA-approved botanical drugs is small; as well as the number of botanical drugs approved for use in Israel. Israeli regulations have adopted principles of the Food and Drug Administration, and of its European equivalent the European Medicines Agency. As winning approval for botanical drugs remains so difficult, most researchers and entrepreneurs focus on developing and manufacturing food supplements instead. Endorsement for dietary supplements is immeasurably easier to obtain.
As a result, today’s pharmacy shelves are filled with long rows of products containing active substances – substances such as lycopene or antioxidant polyphenols – but the products are sold as dietary supplements, not drugs.
Another example, turmeric, has long been recognized as an active agent against non-bacterial inflammations. Several companies sell capsules of turmeric extract as a food supplement. Garlic and onion pills are also sold as dietary supplements. There are various types of algae from which all sorts of active materials are extracted and sold as food supplements.
“Occasionally it happens that from a botanical source, they isolate an active substance that can be used as a drug. Then its chemical structure can be analyzed and then researchers need to find how to obtain an identical but synthetic substance, in case the botanical material it isn’t always available — or merely to work more naturally and comfortably under regulation,” explains Dr. Even-Chen.
For example, the drug Taxol, which is used in chemotherapy for breast cancer, was first discovered in the bark of the Pacific yew tree. To extract enough of that substance, someone would have to plant yew forests across the Amazon. Instead, they identified the molecular structure of the active agent, and today the drug is chemically synthesized without the yew trees.
“The industry in Israel is very creative, and it turns out many studies,” Dr. Even-Chen says in conclusion, “whether the goal is producing ‘botanical foods’ that are spiked with active substances or whether it’s new technologies in the life sciences from the Technion and other institutes.”
Algae – An Oasis in the Desert
Algatech, a biotech firm established in 1998, develops and manufactures active substances from microscopic algae. The microalgae are grown in a closed, sustainable, environmentally friendly system that harnesses the nourishment of desert sunlight.
Algatech is a global leader in the manufacture and supply of one of the most powerful antioxidants in existence – astaxanthin, a natural substance based on a one-celled species of microalgae called Haematococcus Pluvialis. The material is marketed under the brand name AstaPure.
Microalgae are extremely adaptable microorganisms that grow in a variety of locations and are naturally rich in a wide range of healthy and beneficial substances. To date, scientists have identified some thirty thousand different species of microscopic algae that produce unique and potentially useful chemicals. Products based on micro algae have a broad variety of unique applications in the food industry as nutritional supplements, as drugs, and in cosmetics. However, not very many species are being used for practical and commercial purposes due to practical concerns. Commercial growers of microalgae need to find a delicate balance between the necessary growing technology, the available growing location, and the desired final product. The task demands time, capital, and versatility.

As Hagai Stadler, CEO of Algatech, explains, the company was founded by members of Kibbutz Ketura in the Negev who sought an economically viable path compatible with the Zionist vision of settling the Negev and making the desert bloom. The kibbutz wisely found ways of leveraging the desert climate by turning its apparent disadvantages – isolation and active solar radiation – into advantages. Thus, the establishment of APC (Arava Power Company), which has become one of Israel’s largest solar energy companies, as well as Algatech, one of the world’s most advanced factories in the manufacture of active substances extracted from microalgae. The Algatech factory extends across a broad area of closed photobioreactor systems containing more than 600 kilometers of glass tubes, all fully monitored throughout the production process. The company’s initial technology was developed at Ben-Gurion University’s Microalgal Biotechnology Laboratory by Prof. Sammy Boussiba, one of the world’s foremost researchers in the field. Professor Boussiba still serves as head of the laboratory.
The growing process proceeds in several stages, using both clean rooms and open spaces. In the initial “green stage,” the algae benefit from optimal conditions for growth and reproduction. During the next stage, the “red stage,” the algae’s comfort is “violated” as growers introduce more stressful growing conditions. In response to the stress, the algae boost their production of the pigment astaxanthin, which turns the algae red.
Efrat Kat, Algatech VP of Marketing & Sales, explains: “The moment the algae experience stress, they produce astaxanthin, which allows them to survive under extreme conditions. In nature, the algae are found in cisterns and puddles, and the stress comes when the water is gone. The drying algae turn into cysts and create astaxanthin to survive until the next rainy season. Astaxanthin is the same pigment that makes salmon salmon-colored. Studies say that it’s also the reason salmon can survive swimming upstream for hundreds of kilometers without food. Astaxanthin is anti-inflammatory, and it’s one of the most powerful antioxidants in nature. More than 400 clinical and paraclinical studies demonstrate that astaxanthin is good for our health, and research has only begun.”
Kat adds: “Manufacturers of nutritional supplements in more than 40 countries buy astaxanthin from us as a raw material. Our biggest markets are Japan, the US, and Europe. We also sell to large industrial companies, including Fujifilm Holdings in Japan – a huge corporation. Fujifilm decided to make astaxanthin its flagship product, and it launched a line of astaxanthin cosmetics and nutritional supplements. Now it’s one of Japan’s leading companies in that market.”
“Producing natural substances out of algae is complex,” Stadler continues, “You need many capabilities- biological, technical, engineering, processing, extraction, QA +QC, regulatory compliance, patent expertise, marketing channels, and profound market understanding. The process is very involved. It takes time, knowledge and capital – as well as quite a bit of luck. Today, Algatech is working at various universities to develop more substances. In years to come, we plan to commercialize some of the new active substances we’ve developed in recent years.
“Each type of algae is a world unto itself. It calls for its own growing technologies and know-how. At Algatech we’re multidisciplinary, we’re experienced, we have various techniques for growing and processing algae, and most important is our team of specialists – world-class marine biologists, biotechnologists, chemists, operators, and technicians. With all these strings to our bow, we can reach high efficiency and a good R&D success rate for new algae products.”
“Microalgae are where the food chain starts,” Stadler says. “They’re primordial. Over the years they’ve had to compete with bacteria, mold, and other entities to get food, so they’ve developed mechanisms and components that help them stay alive. Some of those components can help humanity meet future challenges and improve the health and welfare of both people and animals.
“In the 2030s, we could be looking at a 40% shortage of protein. But by now we have algae that contain more than fifty percent protein, including all the amino acids humans require. So, some of the world’s major corporations are interested. With advanced technologies, an industry can turn algae into the chief supplier of protein in the coming decade.
“Algatech is a member company of the EU FoodConnects consortium. That’s a €1.5 billion consortium for developing new food sources, and the issue of protein takes a central place there. PepsiCo, Nestlé, Roquette, and Dohler are all in the consortium too.”
During the development process, Algatech carried out projects with the backing of the Chief Scientist at the Israel Innovation Authority. “We owe our successes, to a large extent, to the Israel Innovation Authority, to Ofra Lotan at the head of it, and to its professional staff, for supporting Algatech in recent years. We have to give credit to the Authority’s staff for taking us seriously, and for all they’ve invested in assessing and reviewing our projects. The assistance from IIA is what has led Algatech to the impressive achievements we have made,” Stadler stresses. “This year we intend to launch a new product, algae extract, that tackles fatty liver disease and metabolic syndrome. The product took years of complex, costly development, and our ability to reach this point, just inches from the finish line, is largely thanks to the faith and financial support of the Israel Innovation Authority.
“The processes involved in R&D are not trivial; they demand lots of ability, research effort, and financial investment. Algatech is considered a world leader in microalgae today, and a source of pride in the field of Israeli technology. In the southern Arava, Algatech is a chief provider of jobs and an engine of economic growth. If it’s going to continue expanding as a cutting-edge business with a genuine power to contribute to global welfare – while located in one of the country’s most remote regions – government support is necessary. We hope that the Israel Innovation Authority will continue to help our company in our future research and development projects.”
Innovation in the Operating Room
MediWound is an Israeli biotech company that develops and markets unique products based on enzymes extracted from the stems of pineapple plants – for the treatment of burns and chronic wounds. From fully developing and manufacturing drugs at its sterile production plant in Yavne to directly marketing them worldwide, it is among the few Israeli companies that independently manage the entire value chain for their products. In keeping with the current trend in the medical world, MediWound seeks to replace surgical and traumatic treatment of burns with topical, non-invasive treatment.
NexoBrid, an innovative drug developed by MediWound, makes it possible to treat burn wounds through the removal of injured tissue within hours, all in the patient’s hospital bed and without harming healthy skin. Such treatment saves the patient many operations and potential scars. The drug is likely to play a significant role in mass-casualty incidents where a hospital’s capacity to perform operations is severely limited. In such scenarios, swift medical intervention is critical.
The company’s CEO, Gal Cohen, relates the story of MediWound: “Professor Lior Rosenberg founded the company. He’d been at an international conference where an American doctor, named Gerald Klein, gave a talk about treating burns using enzymes from the stem of the pineapple plant. At that time, such an idea flew in the face of conventional medical wisdom. So Dr. Klein purposely burned himself, and he invited members of the audience to check him after several hours and see what the treatment did. Professor Rosenberg accepted the invitation. The results he witnessed made him want to research this particular enzyme, and from his research, MediWound was established in 2000. For more than a decade, the company has overseen NexoBrid’s development. It’s been a painstaking project, but NexoBrid has proven itself in a series of trials on hundreds of patients around the world. It’s safe and efficient, it’s revolutionary, and we’ve won approval to market it.”
The company’s two flagship products, NexoBrid and EscharEx, are founded on MediWound’s patented enzymatic technology and are considered breakthroughs in medicine. The enzymes are extracted from pineapple plants through an intricate, unique, and controlled process. The products help efficiently break down certain proteins found in injured tissue, without causing harm to the healthy skin. The enzymes can also contribute to lessening the intensity of the inflammation process – a process that, if it persists, can cause scarring. Thus, the enzymes reduce the number of scars among patients.
The company’s vision: Optimal treatment that is swift, efficient, and non-surgical, for patients everywhere.
NexoBrid is a breakthrough because among the products based on botanical proteins, it is one of the few approved for use as a biological drug, and because of the enormous analytical challenge involved in ultimately bringing it to market. NexoBrid has been approved for marketing in Europe and Israel as a novel orphan drug (a drug that only a small number of patients need) for removing dead tissue found on medium to severe burns.
The drug comes in gel form, and a single application removes burned tissue without harming healthy skin. Clinical trials have proven that it significantly reduces the frequency and extent of operations and scars. NexoBrid is expected to serve as an essential tool in treating victims of mass-casualty incidents where currently the hospitals have too few operating rooms and surgeons for simultaneously treating all the burn victims. Already, NexoBrid is being used by hundreds of doctors in treating patients at dozens of burn centers worldwide. The drug has been approved for use by the EMA (European Medicines Agency) and is marketed across Europe, Israel, and Argentina. Numerous health agencies elsewhere are testing it. In light of NexoBrid’s capabilities, the US government has agreed to finance the drug’s continued development in the US and, to be prepared for mass-casualty incidents, it will build an inventory before approval.
The second product, EscharEx, is an advanced drug for removing dead tissue from chronic and hard-to-heal wounds. The drug can aid in the treatment of diabetic ulcers, venous insufficiency ulcers, hard-to-heal wounds, and other afflictions. It is intended for use in conjunction with other existing products in the chronic wound market which require a clean wound surface to achieve complete healing.
EscharEx is designed to treat chronic wounds that cause patients great suffering and even disability. The number of patients with such injuries increases year on year as the population ages and as diabetes and obesity, two causes of chronic wounds, become more frequent. The product has undergone a successful trial on two chronic wound patients. MediWound, with the support of the Israel Innovation Authority, continues to advance the development of EscharEx to obtain approval for commercial use in this growing market.
Aside from those two drugs, the company has begun developing another product (also based on pineapple enzymes), administered via injection for patients who suffer from connective tissue disorders such as severe scarring.
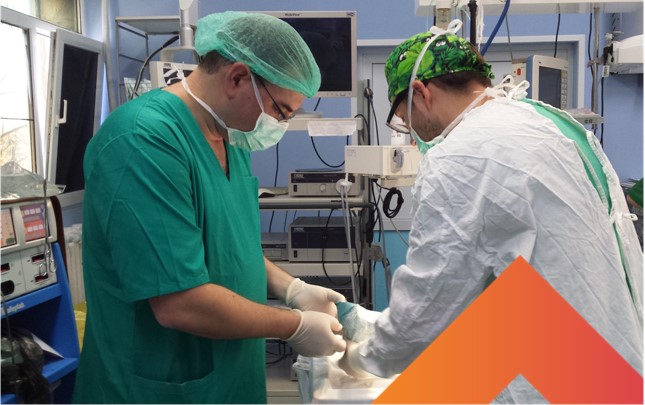
Dr. Yaron Shoham treats victims of burn incidents in Romania using NexoBrid (courtesy)
“The Israeli pharma industry has developed some unique drugs, but NexoBrid is one of the few that have been approved for commercial use. It is truly a global breakthrough in its field,” says Cohen. “All this would be impossible without the assistance and support of the Israel Innovation Authority throughout the years. The Authority assisted us in funding the drug’s development and expressed its trust in the science behind MediWound – despite the challenges of developing a novel drug on an international scale.”
