Two Israeli companies in the field of biomedicine are developing technologies that defy the imagination: Quris creates miniature human organs that enable the safe, quick, cheap, and personalized testing of drugs at a lower cost; Qulab Medical is developing patches that facilitate the prevention and treatment of diabetes.
Dr. Isaac Bentwich, the founder and CEO of Quris.AI, is a physician by training and an entrepreneur by vocation. Quris is the fourth company he has founded and led to global success in the field of AI used in medicine.
Quris’s field of activity sounds like science fiction: a miniature human organ – a one-millimeter brain or beating heart or a third-millimeter liver – immersed in a small quantity of liquid within a small test tube. And not just any organ, but rather one that belongs to a specific person, something called an “organ-on-a-chip”.
The process defies imagination: the miniature organ belonging to a specific person is created from a mere blood sample. When several such organs are combined, the result is a “patient-on-a-chip”.
“It’s amazing”, Dr. Bentwich agrees, “but we didn’t invent it”. The field of organs on a chip has been at the forefront of science in the last decade but it has only matured during the past two years, with the publication of hundreds of articles.
Another step forward occurred 18 years ago with a discovery that won the Nobel Prize – of induced stem cells. In other words, scientists discovered a way to create stem cells not from an embryo, but rather from a mature cell such as blood. This “magic” also contributed to the making of “organs-on-a-chip”.
There is no need today to open the brain and remove a piece. It’s enough simply to take a blood sample, to turn it into a specific stem cell, and to encode it biologically by adding substances that cause a stem cell grown in one test tube to become a miniature brain and another cell grown in a different test tube to become a liver.
“When we talk about having a brain in a test tube, we’re naturally not talking about a brain that thinks thoughts, but it is a specific person’s organ, one that differs from that of another person, and when we drip a certain drug on the brain or the liver, it will react similarly to how that person’s organ would react”, Dr. Bentwich explains.
92% Failure: Developing Modern Drugs
Let’s take a step back and look at how the medical world developed drugs until now. “If we look at the wider picture”, Dr. Bentwich explains, “we see that until now, the entire medical field, and specifically the development of new drugs, failed to take the differences between the individuals into consideration: men are different from women, Caucasians are different from Chinese – we are all different from each other”.
The “old world’s” pharmaceutical industry was based on a kind of funnel: the process started from the scientists’ general understanding of the body’s mechanisms. Whether an antidepressant or an antibiotic, the process involves a study of the human body and an identification of the drug’s objective i.e., how it will benefit a patient’s health. That is how the long end extremely expensive journey of developing a new drug starts in the medical industry.
During the next stage, millions of molecules are scanned in an attempt to assess what best meets every need. Several molecules that are identified as potentially efficient are then examined in greater depth on a culture of cells in the lab. For example, if we are looking to develop an antidepressant, we grow a culture of brain cells in a petri dish. “This method works sometimes”, says Dr. Bentwich, “but it is not accurate. First, because it’s not actually a real brain, but rather just one type of cell without 3D-type integration with other cells.
“The next stage is to locate a molecule that has a chance to work. We drip it onto a petri dish of say, brain-neuron cells, and if it works well, we can hypothesize that it could be developed into a drug and we try to guess how it will work in the human body. Because we can’t administer the drug to humans, we test it on animals. Naturally, this isn’t ideal because a mouse is not a human, but mice do at least have a somewhat similar systemic reaction: an intestine that absorbs drugs, a liver that metabolizes them, a kidney that excretes them, etc.
To what degree is this inefficient? “The answer to that question is the number that constituted my inspiration to found Quris”, says Dr. Bentwich. “92 percent of all drugs that have been successfully tested on animals – fail in clinical trials on humans!”
To illustrate how problematic this is he proposes that we imagine a world in which we want to build a skyscraper. You go to the best contractor, engineer, and architect, and they say: unfortunately, we need to plan and build ten skyscrapers, knowing full well that nine of them are going to collapse – we have no idea which one. The only thing that can be done is to complete the construction of the ten buildings, wait patiently for nine of them to fall, and then increase the price of the remaining building to justify the construction of all ten.
“This is the mechanics and dynamic in the trillion-dollar pharmaceutical industry”, Dr. Bentwich explains. The average cost of developing a new drug is 2.6 billion dollars – simply because it includes all the failures along the way. How much should it have cost? Only 200 million dollars, but it’s expensive because behind each successful drug, there are 5 or 10 failed attempts. “This all means that finding a way that will enable us to make predictions with less than 90 percent errors is something that will make a profound change in this world”.
The innovative development processes became more relevant than ever this past December when the US passed a law that will change this reality: the FDA announced that it is no longer mandatory to conduct trials on animals. “In practice”, Dr. Bentwich says, “the regulator is proposing to look at three areas: Artificial Intelligence, organ-on-a-chip, and stem cells.

The 1001st Drug
“We saw all this happening and said wow – who would have thought that we would be the ones to receive the opportunity to tackle the huge problem of seemingly 90 percent superfluous clinical trials which cost huge sums of money and cause so much suffering to so many patients”, says Dr. Bentwich.
“Science is ready to take on this problem – we have stem cells, organs-on-a-chip, AI, and integrating them can advance finding the solutions by connecting different fields of knowledge. To make this happen, it was necessary to use bio-convergence: an approach that merges different fields of knowledge.
“Already today, we can connect a liver, a blood-brain barrier, a brain, and a heart. We have shown that it is possible to take a drug, to test it on our system, and to make a much more accurate prediction as to which drug will work than was possible with the traditional approach. This will save many more human and animal trials, test failures and, primarily, suffering.
The existing approach in this very new world of “organ-on-a-chip” is to grow an organ in a test tube, to administer a drug to it, and to see whether it works or not. This is done out of an understanding that if it damages the organ, it will probably also harm this person.
Quris operates according to a completely different, AI-based, approach. “We invested 20 million dollars in 3 years to build a platform that is able to conduct thousands of organ-on-chip experiments, later even millions, automatically and cost-effectively”, Dr. Bentwich explains. “This allows us to take 1000 known drugs, put each one on these organs, measure their impact, and enter the data created into an AI system.
“It is as though we are saying to the AI: ‘Here are the results of 500 toxic drugs and 500 non-toxic drugs. And we then ask – will the results of the 1001st drug be similar to the toxic or the non-toxic drugs?”
“The classic development of drugs pretty much ignored the differences between people. Drugs were developed for the average person who doesn’t exist in reality because we are not all identical”, continues Dr. Bentwich. Until today, there was little consideration of human diversity. Now, the FDA is declaring that it will be rigorous in demanding representation of patient diversity in the process of drug development, and it is estimated that this will happen within the next two years”. Quris enables the development of better-suited drugs. Before trying the real clinical trial on humans, it is now possible to test it on numerous organs on a chip, thereby achieving better compatibility.
The Target Audience: Individual Patients and Mega Companies
As befits a company that advocates bio-convergence, Quris employs about 50 people in a range of different professions. Among others, there are biologists, an engineering team that is responsible for the microchips, the miniaturization, the robotics, and the electronics, chemists from the semi-conductor industry, nano-sensor manufacturers, a software team and strong AI team. “We are just like the organs of the human body, none of which can exist on its own, and only the integration of all of them enables this magic to happen”, says Dr. Bentwich.
The fascinating initiative attracted world-quality scientists and professionals, including Nobel Laureate Aaron Ciechanover, the famous biological engineer Robert Langer, former Biogen CEO Michel Vounatsos, Yossi Ben-Amram, the most senior Israeli in the pharma industry, former Pfizer CEO Henry McKinnell, and Chen Barshai who managed the AI teams at Google.
“The Innovation Authority has also been accompanying me for many years”, says Dr. Bentwich. “The Authority was a very significant partner in two of my previous companies. With Quris, we approached the Innovation Authority and received support that was extremely important within the framework of the bio-convergence program. The encouragement, the supportive funding and the evaluators who helped us focus and identify the weaknesses – were all very significant”.
Quris’s business model consists of several pillars. The first is service to pharma companies with the goal of enabling and accelerating a faster, cheaper, safer drug development process that is better suited to human diversity.
The second pillar is a service to wellness consumers: a blood sample is taken from a consumer whose miniature organs are then grown to allow the identification of the appropriate drug for them. “Because the technology is still very expensive, for the next two years the service will be intended for the world’s wealthiest people”, says Dr. Bentwich. “But I expect the cost to drop from 60,000 dollars to a few hundred dollars, similar to what happened with genetic sequencing. As a result, in five or seven years, many of us will be able to produce a “miniature-me-on-a-chip'”.
As part of this service, the stem cells are preserved for future use in organ transplant or to produce actual new organs, something for which there is high demand in the US and elsewhere. This demand is based on the thought that in ten years, it will be possible to grow the miniature organs into larger blocks and to transplant parts of a liver or a pancreas as kinds of human replacement parts”, Dr. Bentwich explains, adding quickly that: “we are not letting that confuse us because we are a small company that is focused on working with pharma with an emphasis on safety, a field in which we are world leaders.
“The Innovation Authority operates in the fields of medicine to advance the development of biochips and supplementary solutions that sparks the imagination, such as organoids. Together with direct investment in companies operating in this field, the Authority strives to create infrastructure centers that serve the entire industry and enhance this field altogether”.
Tzachi Schnarch– Deputy CEO and Head of the Technology Division
A Window into the Human Body
In a world in which one of every ten people suffers from diabetes, one of every three is pre-diabetic, and where nine of every ten pre-diabetics are undiagnosed, it is reasonable to assume that QuLab Medical’s initiative is relevant for you.
QuLab Medical is a startup that engages in biomedical engineering and which seeks to generate a revolution in preventing diabetes, early diagnosis, and management of the disease. All this is achieved with a tiny invasive patch that provides continuous access to metabolic data and enables one to make better and more informed personal medical decisions.
QuLab’s CEO is Dr. Idan Tamir – a trained immunologist. Dr. Tamir was previously involved in several biotechnology companies, managed a technology incubator in the field of medical devices, and founded a family company that develops rapid diagnostic kits.
Dr. Tamir joined QuLab in 2017 after being invited by investors when the company was only a year old. The first step was to transfer the technology from Tel Aviv University and establish an independent company with its own patents.
As far as Dr. Tamir is concerned, the three fields he has worked in – biotechnology, medical devices, and diagnostics – converge perfectly at QuLab. The company engages in the diagnosis of chronic and acute diseases – from early identification of sepsis to diabetes, via technology based on microchips and micro-electronics.
The company’s first product fell under the definition of wearable medical devices – smart sensory patches attached to the skin tissue that monitor biological parameters. The patches include chips and biotechnology tools – enzymes with innovative coatings – and continuous checks of several metabolic parameters simultaneously.
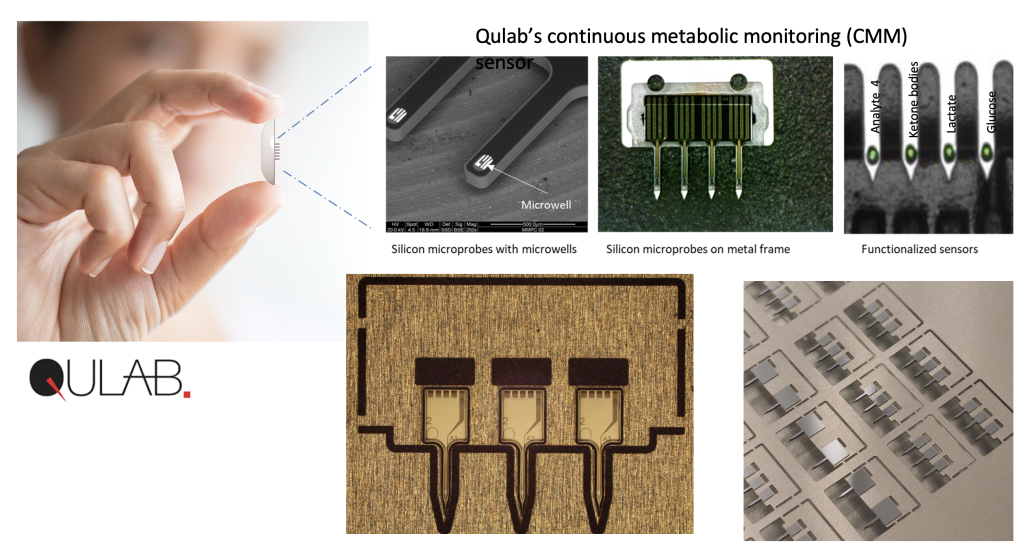

Advantages of the Smart Patch
According to Dr. Tamir, the existing wearable patches on the market have two primary limitations: their relatively high level of invasiveness and the fact that these products only monitor sugar levels.
In contrast, QuLab’s patches contend successfully with both limitations. First, they reduce the invasiveness because the sensors only penetrate 1.5 millimeters into the skin. Furthermore, they don’t only monitor sugar – which is not the sole factor in our metabolic activity – but also other parameters such as lactate (lactic acid) and ketone bodies.
Lactate is an immediate product of sugars’ breakdown. Excessive lactate causes muscle to be less efficient and even damages it. Lactate is a kind of warning signal for the body: rising levels are a sign of distress, maybe as the result of excessively intensive physical activity, distress resulting from internal blood loss, organ failure etc.
The first population interested in continuous monitoring of lactate is sportspeople, who want to achieve maximum muscle use without creating excess lactate. Moreover, the monitoring of the general population has value because it provides indications of medical problems.
Today, hospitals only monitor lactate in a non-continuous manner. QuLab believes that continuous monitoring via their sensory patches will enable early prediction of various acute medical conditions.
Another parameter monitored by QuLab’s patches is what is known as “ketone bodies” – a metric associated with the breakdown of fats. This is a very important metric that should be monitored continuously, among others, in diabetes patients. Enhanced levels of ketone bodies in these patients are an indication of an unhealthy lack of insulin.
Continuous monitoring of ketone bodies is also relevant for the general population i.e., people not yet defined as diabetes patients. This is because changes in the level of ketones may enable to monitor disease development. Early identification will also allow the prevention of diabetes through changes in lifestyle and diet.
In other words, using QuLab’s patches will enable not only to treat known diabetes patients but also to help in delaying or even preventing disease onset among the general population.
From Specific Information – To Continuous Data
QuLab’s intends to begin with a patch that continuously monitors just one metabolite. “The market is saturated with patches that monitor glucose”, claims Dr. Tamir, “so we went for continuous measurement of lactate and we will focus on a solution for hospitalized patients – one that will give physicians the ability to monitor the patient’s situation in a controlled environment and to receive what they lack today: early intervention.”
For example, the transition from a prick in the finger – which provides information about the level of sugar at a given moment – to continuous data, is a huge advantage. Thanks to the continuous monitoring via an existing wearable device, we discovered, for example, that many diabetes patients are unbalanced at night.
“Once we attach a patch that monitors the patient 24/7, we can, at any given moment, see their sugar level and also prevent hypoglycemia and hyperglycemia”. The patch will allow to simultaneously measure levels of sugar, ketones, lactate, and other metabolites.
The benefit involves more than just this specific value – the speed of change in the metabolite level is also critical. One of the reasons to introduce use of the patch in hospitals is that it enables monitoring of the changes in levels of lactate in addition to the continuous monitoring of the patients’ vital signs, thereby yielding significant data about the patient’s condition and enabling physicians quicker detection of any deterioration.
The bottom line is that QuLab’s product will facilitate immediate personal feedback about parallel changes in various metabolites, including sugar and lactate. It will also enable to monitor more parameters and will be less invasive. Dr. Tamir compares the difference between the existing patches for continuous monitoring of sugar and the new patches to the difference between a black and white television with its shades of grey, and a color TV.
How Does it Work?
QuLab is participating in two Innovation Authority programs. The first supports the development of a sensor that will be used for the continuous monitoring of lactate in hospitalized patients for early sepsis diagnosis and other clinical indications.
“About one-third of patients hospitalized in Intensive Care Units in the US develop sepsis”, says Dr. Tamir. “We want to identify them as early as possible to save them from prolonged hospitalization and even death”.
The second collaboration with the Innovation Authority is in the Bio-Chip Consortium, which is dedicated to bio-convergence and in which seven companies and twelve academic institutions take part. Within this consortium, QuLab is combining its technology with the technology of the Israeli company Tower Semiconductors together with other contributions from academia and industry, to create a fusion of data from several sensors simultaneously.
The consortium encourages and facilitates integration between biological technologies and the microchip and microelectronics industries. The fusion between the two fields “overlaps well with QuLab’s operational focus”, says Dr. Tamir. “The collaboration with Tower, for example, gives us the possibility to create reproducible microchips that enable serial wafer-level production, providing the framework for production at very large scales.”
The company’s technology relies on nanotechnology-based microchips. These include an electrochemical sensor fabricated at close proximity to a field-effect transistor (FET), based on nanowire technology. “The entire system requires a company that has the capability to fabricate, at nanometric resolutions, different elements from silicon including miniature transistors localized at the tips of microprobes.
QuLab has passed several developmental milestones in preparation for serial production and is working on the capability to integrate its sensor chips into the patches, enabling minimally invasive intradermal sensing.
“We are at an advanced stage of animal trials which are mainly being conducted on pigs”, Dr. Tamir explains. Because the patches have minimal invasive sensors, they don’t harm the animals. The company has already begun its first human trial which will primarily check the safety of inserting the sensors into the skin. If a subsequent clinical trial conducted on humans is successful, a request will be submitted for regulatory approval for the product’s use.
A Periodic Checkup for the Body
The product will initially be approved for use by medical staff in hospitals and the data gathered will be collated and processed. Following this process, the medical indications for using the product will be expanded. The intention in the next stage is to also make the tool accessible to the general public. The patch is intended to be a wearable device with which a person can move freely and even shower. The planned range of use is about two weeks – in accordance with the industry’s existing standard.
QuLab’s system transmits the data via ‘Bluetooth’ from the chip to the cellphone. Because the raw data received from the sensors about the metabolites is difficult to interpret by the lay user, the data must be transferred for immediate automatic analysis via a dedicated AI-based algorithm. This will enable to present the bottom line to the users about what they are doing right or wrong in relation to diet and physical activity and to provide recommendations.
Although QuLab’s current example includes three metabolic metrics that are, in principle, received from three parallel sensors, six or even more sensors can be inserted under the skin using the same-sized patch, with each one simultaneously monitoring a different parameter or metabolite. The platform itself can also be implemented in the future for additional indications. In other words, the capability of continuously and simultaneously measuring different biological parameters will enable to monitor and warn, not only about diabetes, but also about other diseases.
QuLab has a dual vision: on the one hand, the company seeks to help people contend with a given chronic situation. On the other hand, the aspiration is to prevent the creation of the chronic situation in the first place. As Dr. Tamir explains: “Even if people have a genetic tendency to develop diabetes, we predict that it will be possible to significantly delay its progress and to give them additional years of health.” “Ultimately, a large part of the existing approach to health is the correct and sensible conduct of each and every one of us with our individual metabolism, body, and genetics. Just like a car has microchips that monitor risks and undergoes a regular checkup that prevents faults, our body will also undergo continuous monitoring to warn us of potential problems. This will enable us to take better care of our bodies and live a healthier and longer life.
