Printing a cornea, engineering a heart, growing meat, and helping the paralyzed walk – this may all sound like science fiction but it’s happening right now in laboratories in Israel engaging in Bio-convergence. Four scientists explain what happens when you combine biology, engineering, medicine, physics, and chemistry.
“We represent Bio-convergence’s message of integration”, says Prof. Shulamit Levenberg about her role as Dean of the Biomedical Engineering Faculty at the Technion and Director of the recently inaugurated Center for 3D Bioprinting. Prof. Levenberg joined biomedical engineering 15 years ago after completing a post-doctorate degree at MIT with Prof. Robert Langer, one of the world’s leaders in the field of tissue engineering. One of Prof. Levenberg’s noteworthy laboratory achievements is the repair of injured spinal cords in rats by implanting engineered tissue from human stem cells.
“During my post-doctorate in Prof. Langer’s lab, I first engaged in the field of tissue engineering”, says Prof. Levenberg. “I was fascinated by the connection between engineering and biomedicine and was therefore glad to accept the opportunity offered by the Faculty of Biomedical Engineering at the Technion. This is a winning combination – people with background in both science and engineering who harness all their knowledge to lead a change in the world of biology and medicine.
“I see the same thing in biomedical engineering curriculum – the combination of broad engineering knowledge together with medicine, biology, physics, chemistry, computers and mathematics. We want to provide students with superb training so that they can be the best in their fields.
“If we focus for a minute on the field of tissue engineering, the idea is to create tissues for implants that induce regeneration i.e., regrowth. We aspire to create pieces of 3D tissue in the lab that we can insert into an area of defective tissue or organ and repair it. The field has undergone a dramatic transformation from 2D cultivation of cells on a petri dish to 3D growing on a growth platform. The ‘scaffolding’ can be biodegradable i.e., break down over time, and the cells eventually secrete the same natural matrix, producing tissue.
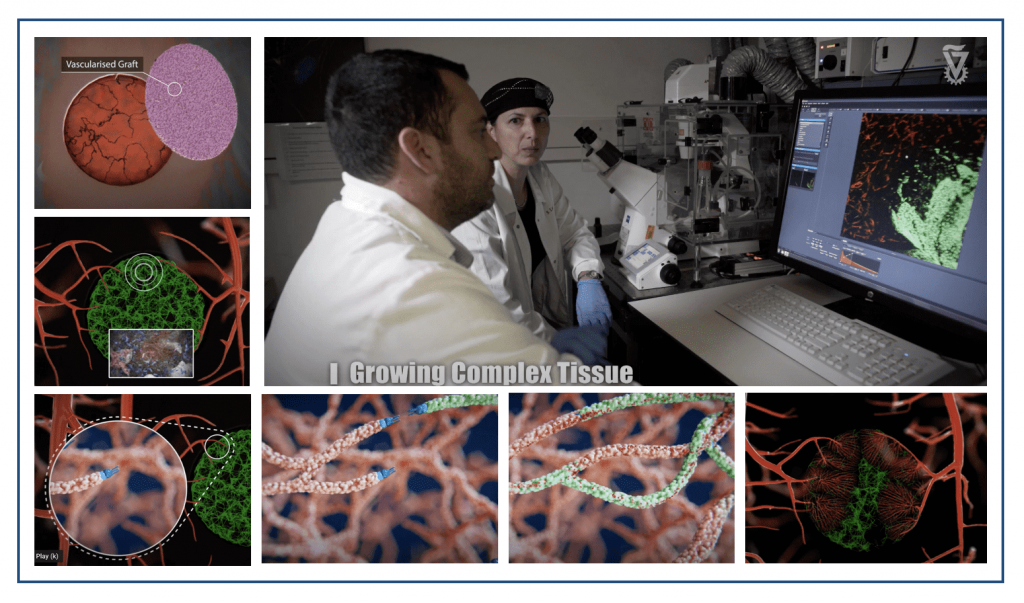
“In order to create pieces of tissue, we combine stem cells capable of division and differentiation together with the 3D scaffolds, and sometimes make use of bioreactors in special environmental conditions that allow us to grow the tissue. One of the challenges that we are focusing on, in this field, is how to create a network of blood vessels (vascularization) in the tissue. After creation in the lab, the tissue then needs to survive and function within the body and therefore needs an immediate supply of blood and nutrients.
“We showed that creating a network of blood vessels in the lab-created tissue helps connect this tissue after implantation. We apply this direction of 3D creation of vessels inside the tissue when creating muscle tissue – for example, abdominal wall muscle or heart muscle.
“We have proved that we can create tissue that will assist with the regeneration of neurons in the spinal cord and enable paralyzed animals to walk again. We are also funding projects to reconstruct bone with soft tissue and blood vessels to create multi-layered tissues that are in need of large-scale and comprehensive reconstruction. For more complex tissue when there is a need to make specific adjustments to the injured area, we turn to the field of bio-printing that provides us with a tool to create more complex and precise personalized tissue.
“The major obstacles when bringing the field of tissue engineering to the clinic is that of size and the need to deliver nutrients to large tissues. The challenge is to engineer a large tissue that can be grafted in the body and function properly.
“The transition to the medical clinic involves engineering challenges and the integration of Bio-convergence therefore becomes extremely significant and even necessary when it comes to addressing more complex developments. The field of biomedical engineering, that encompass tissue engineering, leads this integration and it is important to mention that the emphasis is on practical application. For example, Aryeh Batt founded a company that leads the commercialization of clinical use of tissue printing. We want to see more companies take the field in parallel directions, thereby leading to better advancement in the clinic. In our tissue engineering research we have conducted experiments on animals and now need to take the next step to clinical trials, but we must first focus on several other developments that will provide a solution to the dimensional challenge.
“The tissue is highly complex and organized – it is a collection of cells each of which is organized for a specific role within the tissue. We are trying to decipher the way cells organize and arrange themselves to create the tissue in the lab that will best integrate into the body. One of the main challenges is to discover and imitate the cues of the cells in the body and their knowledge in how to come together and form a tissue.
“Throughout my research years I have benefitted from the incentives provided by the Innovation Authority towards projects with potential for commercialization. For example, we took a major step towards commercialization when we founded Aleph Farms – a company based on our tissue engineering technology that is attempting to create cultured meat tissue. The idea was that if we know how to create a piece of muscle for implantation, then maybe we can similarly create a piece of cow muscle that can be used for food. Commercialization is driving us as researchers and the collaborations in this area are of great importance.
“Our vision can be divided into 3 levels. The first is to advance research, to find new discoveries and to develop the world of science, engineering, and human health. The second level is to train a new generation of scientists and to watch them integrate in and contribute to research and industry. The third level is to see how things can be practically applied in medicine”. The interviewees featured below can be regarded as examples of the fulfillment of Prof. Levenberg’s three-fold vision in the field of Israeli Bio-convergence.
Anya Eldan, VP and Head of Startup Division at the Israel Innovation Authority: “Successful repair of damaged tissues and organs requires a combination of multidisciplinary forces that brings together engineers and biologists, different fields of science and engineering, materials science, and unique 3D printing. Today, the field of Bio-convergence enables us to develop innovative tissue engineering technologies based on innovative 3D printing technologies which in turn facilitate the “building” of new organs at single-cell resolution using new nanomaterials. Israel is characterized by leading research and pioneering companies in this field enabling Israeli entrepreneurs to fuse disciplines and build integrative development teams with experts from different technological backgrounds. This is our advantage as a small country – everyone knows everyone else”.
Would you like a printed heart?
“When I registered for university, I heard that they were opening a new course at Ben-Gurion in bio-technology engineering – a combination of engineering and biology. I thought to myself: there are plenty of electrical engineers, but not so many biological engineers”, says Prof. Tal Dvir from the School of Molecular Cell Biology and Biotechnology, Department of Materials Science and Engineering, Center for Nanoscience and Nanotechnology and Sagol Center for Regenerative Biotechnology at Tel Aviv University about the beginnings of his academic career. “I am very happy that my background is multidisciplinary – I studied engineering, chemistry, biology, and computers. It gives me a basic understanding and I am constantly collaborating with doctors, chemists, and biologists. To achieve something advanced, original, and innovative, you need a combination of disciplines.
“My laboratory works on tissue engineering, a field in which cells are transformed into functioning tissue. It is important to understand that tissues in the human body are not just about cells and that the biomaterials that cause them to work together are no less important. We study and develop biomaterials like this, insert cells into them, and cause them to transform into functioning tissues. To do so, we must adapt the biomaterials to the final tissue we want to engineer.
“Although we gained a fair level of publicity over our progress with heart tissue, we are working on a diverse range of other tissues and organs. For example, we recently founded a company that is based on research we conducted on the construction of a spinal cord implant from the patient’s stem cells and biomaterials, and on rehabilitating his movement and ability to walk. Our vision is to treat paralyzed patients in the clinic. Another example of our work is an implant that releases dopamine and regenerates the brain in cases of Parkinson’s Disease. We are also working simultaneously on the bowel, retina, and kidney. Each organ has its own challenges.
“Three-dimensional printing has a huge advantage over the other approaches to tissue engineering. It enables us to position different cells in different places. For example, a heart muscle is made of heart muscle cells that contract but also of a large number of blood vessels that need to nurture the heart muscle tissue. In 3D printing, we simply decide where the blood vessels or heart muscle cells will be placed and initially print a tissue and only subsequently the whole organ.
“Each project is at a different stage of implementation. In the heart project, the current goal is to implant the 3D-printed heart in rats during the next 12 months. In the spinal cord project, the company (Matricelf) would like to treat patients in 2 years. We have achieved 100% success with small animals – more than 20 animals are now walking well after injury – but animals and humans behave completely differently.
“From a regulatory perspective, it will be relatively easy to obtain FDA approval for a therapeutic approach such as this for the spinal cord because no damage can be caused to people who are already paralyzed. It is more complex with other tissues and so we are also working on printing tissue that can be injected instead of transplanted, thereby preventing the need for invasive treatment. The goal now is to recruit additional researchers to develop other such technologies and to construct tissues and organs that will regenerate the injured ones.
“Our research is transdisciplinary. We know how to do some of the things by ourselves while for others we need experts. We already have many productive collaborations. Examples of these are Prof. Benny Dekel from Sheba Hospital with whom we are engineering parts of the kidney, and Dr. Aya Barzilai and Prof. Adiel Barak from Ichilov Hospital who are working with us on 3D printing of a retina. These projects are expressions of both the medical need and engineering knowledge and capabilities.
“In the field of biomaterials and tissue engineering we are contending with much more modest budgets than in other areas. Nevertheless, our research in Israel is at a very advanced stage and is being conducted by excellent scientists – from Prof. Smadar Cohen at Ben Gurion University with whom I completed my doctorate, Prof. Shulamit Levenberg from the Technion and many others – and is not inferior to any research being conducted at universities in the US”.
Causing all Technologies to Work Together
“We do things in our labs every day that until recently were considered science fiction”, says Aryeh Batt, CEO of Precise Bio that develops printed tissues for human implantation. “We have developed a printing technology with unique capabilities in the fields of industrial 3D, plastics, and primarily, in the field of biology. Experts I presented this idea to five years ago told me that it was impossible, yet today we already have tissues that have undergone pre-clinical tests and that function excellently in animals. Precise’s 3D printed tissues have the integrity that can be implanted in humans.
“To found Precise, I joined up with two of the world’s leading experts in the field of tissue engineering – Dr. Anthony Atala and Prof. Shay Soker from Wake Forest, who head the world’s leading institute in the field of regenerative medicine”, Batt explains. “Already 20 years ago, Atala was the first person to implant a lab-engineered organ in a child. Today that child is 25 years old.
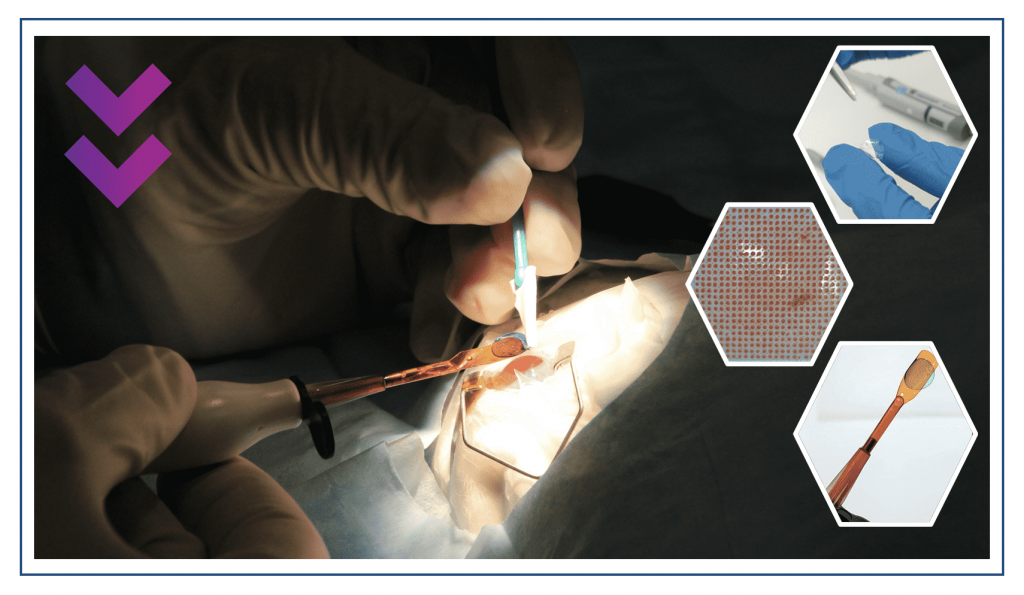
“The Wake Forest Institute for Regenerative Medicine studies all aspects of regenerative medicine including cell division, biological materials, bioreactors and actual implantations. At Precise, we combine all the disciplines needed to create tissues for implanting. It took us quite a few years until we reached the stage of a functional tissue and we were the first company to implant human cornea tissue in a process we define as 4D bio-fabrication – a combination of 3D printing and a biological production process.
“It’s not all about printing. The printer is the production tool, but the process begins with the cells themselves – human cells that, together with other biological materials, make up the tissue. These need to undergo a biological post-printing process. After the printer arranges them in the desired anatomical structure there is a maturation phase. This process transforms the product emerging from the printer from a collection of cells into a functioning tissue and is performed entirely inside a bioreactor.
“Only a few companies worldwide know how to integrate biology with printing. Tissue engineering requires more than just a 3D printer with biological certification but also close interaction between the printer, the cells, and biomaterials enabling the printer to fabricate the printed tissue as it is in the human body. We are unique in this respect and have signed an agreement with a large multi-disciplinary corporate in the field and are presently at the stage of signing a development agreement with several other multi-national companies in the global pharma and medical device sector.
“The company operates two development centers – one in Israel and the second in North Carolina. Most of the workers are from the biology field but we also have a very strong engineering body with disciplines other than biology: physics, optics, software, and engineering, who developed our unique printer. If we’re talking about Bio-convergence, then Precise supplies all the relevant technologies and causes them to work together.
“Our current focus is on ophthalmology, but our platform and technology can also be used in other fields such as cardiology, orthopedics and others. The focus on eye tissue stems from the fact that this is a field with relatively simple regulation that knows how to adopt new technologies. Furthermore, unlike organs where it is important that the tissues come from the patients themselves, cornea tissue doesn’t require compatibility because it does not activate the immune system.
“In the coming year we will progress from the pre-clinic phase to clinical trials with implantations of corneas and other eye tissues, becoming the first company to do so. We have cornea tissues that are aimed at replacing the existing solution of cornea transplants from dead people.
“Cornea transplant is currently one of the most common transplants in the western world. With our solution, instead of waiting for someone to die, we simply print a human cornea in ten minutes that has both quality control and mechanical traits convenient for the surgeon.
“Amazing work is being done in academia but they are 10 years away from clinical application. In contrast, we are bridging this gap and producing things which reach the doctor as a tissue that can be implanted in a patient. Here too, we are collaborating with the world’s leading consultant physicians in the field of eye medicine.
“It took 3 years from the moment we started working on the cornea until we had a functioning tissue, an achievement that is considered as extremely fast in the field of medicine. It took us just a few weeks to shift from one type of tissue to another in the ophthalmology field. Our knowledge and developments now enable us to move from tissue to tissue and from one field to another, serving as a platform for a variety of unmet therapeutic needs.
“One of the unique things about our technology is its capability to print inside the human body, for example, to enter the spine in a minimally invasive procedure and print cells inside the body itself. So far, this has only been done in a lab experiment and not in animals, but the technology exists.
“We have an excellent connection with the Innovation Authority because they have an exact understanding of the needs of companies like ours. We are at the post-academic stage and ahead of large companies, and the Authority assists us in finding ways to participate in its programs.
“There is a lot of academic endeavor in Israel in the field of Bio-convergence – for example, Prof. Shulamit Levenberg and Prof. Tal Dvir who are doing amazing work – and the Innovation Authority wants to bring these parties together. As a company that can cultivate other companies, we view this as a national task. Acquiring more knowhow in these areas will enable these companies to grow even more.
“The Authority’s mandate is to support exceptional innovation and developments while venture capital funds look for low risk. We are obviously high-risk, simply because no one has done this before. Amazing things are being done in academia, but they don’t have shareholders. We do, and we choose our battles accordingly. Naturally we would like to print kidneys and a heart – and in another 15 years we will – but as a company that needs to show profit, we chose the cornea and other relatively simple tissues because that’s what is realistic to expect regarding short-term market delivery”.
The Steak that Grows in the Lab
Another example of a technology developed in academia by leading researchers and then transformed into a commercial company with the support of the Innovation Authority is Aleph Farms. The company is based on technology developed in Prof. Shulamit Levenberg’s lab. Unlike Prof. Levenberg’s work on applications in the medical field, Aleph Farms focuses on a different direction – production of cultured meat for food consumption that will prevent the slaughter of animals and harm to the environment.
Didier Toubia, a food engineer and biologist by training, is the co-founder and CEO of Alpeh Farms. “There are presently quite a few challenges in the way meat is produced”, he explains. “The primary challenge is the use of natural resources, especially for beef production. All the customary growing methods require large water and land resources and emit large quantities of greenhouse gases including methane. This also requires transportation and slaughterhouses that further harm the environment. Furthermore, the same dependence on water, land, and the climate dictates that cows cannot be grown everywhere and many countries in Asia and the Middle East (including Israel) are forced to import their cattle.
“Public health is a further challenge. We are witnessing increasing disease among animals grown in a concentrated manner, for example swine flu, bird flu, and mad cow disease. These epidemics threaten both public health and the balance of the food system. Large quantities of antibiotics are used to combat the diseases leading, among others, to the deaths of more than 700,000 people every year due to resistance to antibiotics. The antibiotics cause the bacteria to mutate and develop resistance and they then infect humans for whom the existing medications are ineffective. It is not surprising that the World Health Organization has defined the issue of antibiotics resistance as one of mankind’s largest challenges.
“The third underlying problem in concentrated industrial farming is the conditions in which the animals are raised and slaughtered. More and more consumers are becoming sensitive to the cruelty to animals associated with these methods.
“We offer a new method of meat production. In contrast to vegetarian meat substitutes, our approach is not to create an alternative to meat but rather, to produce cultured meat grown outside the cow. In other words, we do not replace meat with something else but rather merely replace its production process.
“Our goal is to reconstruct a natural process of beef tissue renewal. We isolate cells that have the ability to build new muscle tissue in a cow in order to replace old muscle tissue and transfer these cells to external means that simulate the conditions in the animal so that they continue to grow muscle tissue as if they were still located in the animal’s body. This muscle tissue is, essentially, meat. In this way, more and more meat can be produced from a few cells without the need to raise and slaughter the animal.
“The process we developed enables us to grow a steak in about 3 weeks instead of 2 years in a normal cow. In addition to shortening the time frame, we need far fewer natural resources and energy. The growing process takes place in a closed system, without pollution or antibiotics, and we prevent any problem of cruelty to the animal.
“Aleph Farms was founded in 2017 based on the transfer of technology from the Technion and with the collaboration of the Innovation Authority and Strauss as part of ‘The Kitchen’ incubator. Thanks to the access to the Technion’s laboratories and the cooperation with Prof. Levenberg, we have taken tissue engineering – a scientific technology developed with a medical orientation – and adapted it to the food sector.
“We graduated from the incubator in June 2019 and thereafter continued to receive regular R&D support from the Innovation Authority. After working for many years with the Authority, I am very appreciative of its activity and the fact that it succeeds in renewing and adapting itself with innovative solutions and approaches. Their approach is extremely logical, professional, and reasoned. Both in the previous field I engaged in – medical devices – and the food sector, I view the Authority as having an important role in building the country’s infrastructure. We regard the Authority as a full partner that shares the common objective of advancing this industry in Israel. It just wouldn’t be happening without the Innovation Authority.
“Other companies are also focusing on the cultured meat industry – 3 in Israel and about 40 around the world. Nevertheless, none of them has yet launched an actual product. What characterizes Aleph Farms is the combination of 3 unique aspects. The first is the focus on growing a whole piece of meat, such as a steak rather than a hamburger. The second aspect is a unique source of cells that possess the capability to rapidly reproduce without genetic engineering. The third aspect is the infrastructure for large-scale production required in the world of food, alongside the many patents and knowledge we have developed.
“Our fundamental challenges are to penetrate the market, to reduce production costs, to enable large-scale production, and to meet the high consumer expectations. We need to supply a product of high nutritional and culinary quality and this necessitates a combination of high-level cell biology on a large scale and a knowledge of bioreactors which are the equipment for growing cells, and food engineering. The requirements for a final food product are completely different from those of a finished product in the medical industry.
“Part of the development involves the complex issue of a growth medium providing the cell nutrients that will be both efficient and low-cost. In principle, the goal is to reconstruct the surroundings and nutrients that nurture the cells in the body, without any animal content i.e., only the cells themselves are animal-based.
“In 2018 we were the first company to go public with a piece of steak grown outside a cow. We are supposed to complete commercial development of the first product by the end of 2020. Looking forward to 2021, we hope to make the transition to production using our unique platform that will allow to produce large quantities at a reasonable price. It will take about 2 years to optimize the process regarding both cost and environmental aspects.
“We are the first company in this field to publish goals relating to transition to zero-level carbon emissions in meat production by the year 2025. In the wake of the Corona crisis, many European countries have decided to provide added impetus to a green economy, and we are branding Aleph Farms as a leading company in this field.
“We believe that cultured meat will constitute a substitute for meat produced in animals. Production of industrial slaughter-based meat will decrease and there will be a gradual return to more organic methods. This will occur due to pressure on two levels: consumer and national.
“We must ensure that meat remains available and accessible to everyone and that economic development is accompanied by sustainability. A cultured meat industry can support an improvement in quality of life and economic growth while continuing to provide jobs and helping agriculture make the transition to zero greenhouse emissions.
“The idea of cultured meat is also important from the food security aspect. Most of Israel’s meat is imported, either before or after slaughter, because you cannot easily breed and grow cows in the Middle East weather. Meat consumption rises dramatically during times of crisis – in March for example, with the beginning of COVID-19, meat sales increased 73%. So, when there is a crisis that closes the borders, meat becomes a strategic issue. One of the major advantages of “cultivated” meat is that it can be grown anytime and anywhere independent of local natural resources or the climate – we have even grown meat on the international space station. We believe that from a strategic point of view, the State of Israel must invest in its food security including in the local meat industry.
“Beyond the local strategic importance, I regard cultured meat as a way to develop agriculture in the Israeli desert. Food security and development of the Negev region in Israel stood at the center of David Ben Gurion’s and our other forefathers’ vision. My dream is to continue the national agricultural revolution: the kibbutz movement will be followed by cell agriculture”.
